Abstract
Clonal hematopoiesis (CH) is a common age-associated phenomenon of specific somatic clonal changes in blood cells. CH has been found to increase the risk of hematopoietic malignancies and cardiovascular disease, as well as an assortment of other chronic medical conditions. DNMT3A, which encodes a de novo DNA methyltransferase, is the most commonly mutated gene in CH and has a mutational hotspot at p.R882. Here, we used CRISPR knock-in to engineer primary hematopoietic stem and progenitor cells (HSPCs) from umbilical cord blood (UCB) with DNMT3A p.R882H in order to evaluate the impact of this mutation on cellular phenotypes.
To model the R882H mutation, we employed electroporation assisted transduction using Cas9-gRNA RNP along with single-stranded AAV6 as a homology donor. In addition to the desired amino acid substitution, we inserted a self-cleaving fluorescent marker under control of the endogenous DNMT3A promoter. With this construct, we achieved high transduction efficiency (range 39-51%) of UCB CD34+ HSPCs. We then used FACS to isolate mCherry+/BFP+ homozygous-edited CD34+ cells for culture and downstream experiments.
Following a week of culture in HSPC expansion media (SFEM II with Stemspan supplement, SR-1, & UM-729) and a week of culture in monocyte differentiation media (IMDM with 20% FBS, SCF, M-CSF, IL-3, & FLT3L), we performed negative selection to enrich for CD14+ monocytes without CD16+ depletion.
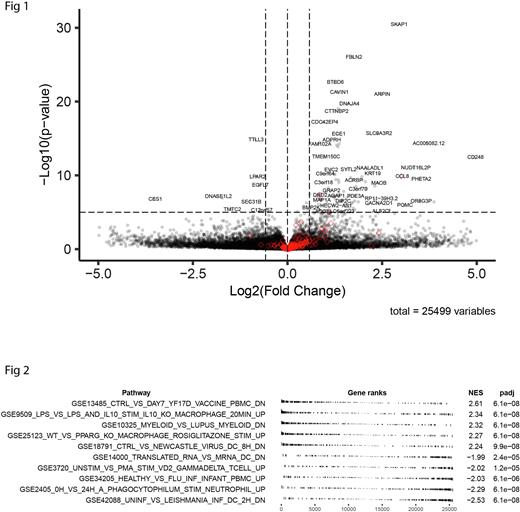
Given that the R882H mutation has been found to associate with atherosclerosis, we then asked if the transcriptome of the R882H population showed any differences in immune signaling pathways at baseline. We therefore performed bulk RNAseq on unstimulated cells (N = 3 biological replicates) and analyzed results with DESeq2. Comparing R882H samples to isogenic wild-type controls revealed 304 differentially expressed genes (FDR < 0.05), including higher expression of CCL8, a monocyte chemoattractant, and MAOB, a mediator of myeloid IL-1β expression and mitochondrial ROS production in response to NLRP3-activating stimuli (Fig 1).
Pathway enrichment analysis was also suggestive of a highly primed immunologic posture in the R882H cells. Examination of the MSigDB Immunological Signatures (V7.5.1) pathways with the highest and lowest normalized enrichment scores showed a pattern consistent with myeloid cell activation (Fig 2). Among the most highly enriched was a set of genes with lower expression in myeloid cells of control subjects compared to those from patients with lupus (GSE10325, NES = 2.32, padj = 6.1e-08, red diamonds in Fig 1). Also enriched were genes with lower expression in pre-vaccine PBMCs vs. PBMCs 7 days after subject vaccination with the attenuated yellow fever 17D vaccine, which is known to generate a strong innate immune response (GSE13485, NES = 2.61, padj = 6.1e-08). Other enriched pathways included genes upregulated when macrophages were stimulated with LPS compared to LPS plus IL-10 (GSE9509, NES = 2.34, padj = 6.1e-08), genes upregulated in WT compared to Pparg knockout macrophages after rosiglitazone treatment (GSE25123, NES = 2.27, padj = 6.1e-8), and genes downregulated in healthy dendritic cells compared to those infected with Newcastle virus (GSE18791, NES = 2.24, padj = 9.9e-08). In line with these, there was also de-enrichment of genes that are upregulated in PBMCs from healthy donors compared to PBMCs from infants with flu (GSE34205, NES = -2.03, padj = 6.1e-6). The preponderance of significantly enriched gene sets describing myeloid activation across numerous contexts suggest that monocytes with R882H are at baseline immunologically primed, even in the absence of a specific insult.
Prior research has demonstrated severe myeloid inflammatory dysfunction in DNMT3A-mutant cells, but these studies often rely on provocative stimuli such as LPS or have used samples taken from patients with numerous comorbid conditions. In this study, we used high-efficiency CRISPR knock-in to introduce DNMT3A p.R882H into primary UCB HSPCs that we subsequently differentiated into monocytes. In doing this, we showed that this mutation may cause a dysregulated immune response in myeloid cells prior to any inflammatory insult. Furthermore, the techniques used in this study can be extended to examine other CH mutations, various hematopoietic lineages, and responses to exogenous stimuli.
Disclosures
Bick:TenSixteen Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Ferrell:Incyte: Research Funding. Savona:Geron: Consultancy; Sierra Oncology: Consultancy, Other: travel expenses; Ryvu Therapeutics: Consultancy, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; ALX Oncology: Research Funding; Astex Pharmaceuticals: Research Funding; Taiho Pharmaceutical: Consultancy; Takeda: Consultancy; AbbVie: Consultancy, Other: travel expenses; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: travel expenses; Incyte Corporation: Research Funding; Novartis: Consultancy; Forma: Consultancy; Karyopharm Therapeutics: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Other: Travel expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal